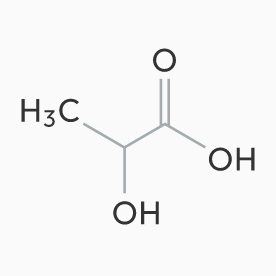
“Polylactic Acid for Medical application” is manufactured from our pharmaceutical-grade Lactic Acid under our excellent technology and strict quality control. We are engaged in this PLA business activity categorized under two kinds of applications: “Medical device” and “DDS formulation”.
Being different from commodity PLA, the specifications for both of the two polymers are set following each customer’s request based on quality and property.
Please feel free to contact us regarding your quality requirements for your requested polymer.